Pharmaceutical Drug used in soap. - ppt download
By A Mystery Man Writer
Description
LYE A strong alkaline solution or solid of potassium hydroxide or sodium hydroxide, made by allowing water to wash through wood ashes. It is used to make soap and drain and oven cleaners. Chemical formula: KOH or NaOH.
Pharmaceutical Drug used in soap
A strong alkaline solution or solid of potassium hydroxide or sodium hydroxide, made by allowing water to wash through wood ashes. It is used to make soap and drain and oven cleaners. Chemical formula: KOH or NaOH.
Sodium Hydroxide for Soap Making. RM 20/kg. To order: SMS/ Whatsapp: FB:
They strive to have the highest quality of cosmetic ingredient and at lowest rate possible.
Shea Butter- Has unique healing properties. High in triglycerides and fatty acids. The high fatty acid of shea butter also makes it an excellent additive to soap. It contains vit A and E. Coconut Oil- Healing ingredient used in soaps to produce a luxurious lather. Ingredients. Olive, Coconut, Palm and Castor Oils, Shea Butter, Cocoa Butter, Sodium Hydroxide (Lye), Mountain Spring Water, goat milk. No fragrances whatsoever.
Potassium Hydroxide is hygroscopic which is it attracts moisture, so be sure to keep it in a sealed container in a cool dry place. it can attract enough moisture from the air in the room to turn completely liquid. Always add your lye to your water. Adding water to lye can cause a volcano-like reaction. wear goggles that protect your eyes from all sides because alkali burns can cause blindness. Always wear an apron and gloves. Use rubber dish gloves. Use a heat safe container to hold your lye water, and use silicone or stainless steel tools to stir (no wood).
Industrial-scale production ; continuous process. Smaller-scale production ; batch process. There are two ways of making soap: Cold process ; under condition of room temperature. Hot process ; under condition near the boiling point.
PROCESS OF MAKING SOAP HOT PROCESS COLD PROCESS MOLD FOR INDUSTRIAL SCALE PURIFYING AND FINISHING
Commonly, the soap makers would used hot process method if they wanted to minimize the cure time to only three day air dry process. These two methods can be used for producing soap especially those who love to make handmade decorative soap. The glycerin will be left in the soap while the reaction take place for many days as soon after the soup is poured into the molds. During the hot process, the glycerin remain in the soap,however when it is applied to a high temperature,the reaction is already finished in the kettle, even before the soap is poured into the molds. In cold process of handmade soap, it uses extra fat rather than production of soap in industry. The fat is used (superfatting) to absorb the alkali, and the glycerin remained act as moisturizing agent. If the glycerin is left wet, it will cause the soap to be more softer and and less resistant mushy. Besides that, it is preferable to add lots of oil and have excess fat rather than to add lots of lye and have excess lye. Excess glycerin can be found also in hot process method. Glycerin soap can also be created by adding glycerin to the soap. It can be observed that superfatted soap is more suitable for skin however, if the fat is added too much in quantity it can leave a greasy feel to skin. Essential oils such as jojoba oil, shea butter and olive oil will remain intact and escape saponification when all lye has been consumed. Lye discount is a process where the soap makers are using less alkaline in superfatting process.
Hot process must saponifies the oil entirely before poured into the molds. The fat and hydroxide are heated and blended together at about °C which is below the boiling point until the saponification is complete. This process is the best way to use up the essential oils that cannot survive in cold process. This is just the fact that saponification has already undergo before addition of essential oils. The temperature of soap rises during saponification and numerous essential oils cook out and remain with no scent. Another benefits of hot process is it preserves the colour that we used. Hot process is more quicker than cold process method. The soap will harden and can be cut on the same day. It can be much faster by putting the soap in the refrigerator or freezer.
After hardened the soap, it will be poured into molds and in long silicon bar and cut into individual portions or poured into individual molds. Purification. At industrial scale, the soap is further purified to eliminate excess glycerol,sodium hydroxide and other compounds in the fully boiled process by boiling the crude soap curds in water and then use salt to precipitate the soap. This process proceeded in spray dryer and then into vacuum dryers. After compacted into tiny pellets or noodles, it is now ready for soap finishing which is by converting raw soap pellets into product. Fragrant and other materials are blended together in a mixer. Discharged mass will go into a refinery, vacuum chamber,cut into respective length and turn into packages. Triclosan or triclocarbon can be added in actibacterial soap.
to ensure complete melting of the fat being used. safe to use after about 12–48 hours, but is not at its peak quality for use for several weeks. Lye(sodium hydroxide) or potash(potassium hydroxide). requires exact measurements of lye and fat amounts and computing their ratio. Historically, lye used in the cold process was made from scratch using rainwater and ashes.
looks up the saponification value for each unique fat on an oil specification sheet. This value is used to calculate the exact amount of potassium hydroxide to react with the fat to form soap. Excess unreacted lye in the soap will result in a very high pH and can burn or irritate skin. In the process, the lye-fat mixture is mixed until the two phases (oils and water) are fully emulsified.
There are varying levels of trace. Depending on how additives will affect trace. After much stirring, the mixture turns to the consistency of a thin pudding. Trace corresponds roughly to viscosity. Essential oils and fragrance oils can be added. The batch is then poured into moulds. Mostly, soap molds are made of silicone or various types of plastic, although many soapmaking hobbyists may use cardboard boxes lined with a plastic film. cold-process soaps are typically cured and hardened on a drying rack for 2–6 weeks before use.
MANUFACTURING OF SOAPS & DETERGENTS
Bar soap. Powder detergent. Liquid detergent. Packaging process.
made from fats and oils. their fatty acids which are reacted with inorganic water-soluble bases. The raw materials may be pretreated to remove impurities and to achieve the color, odor and performance features desired in the finished bar. The main sources of fats are beef and mutton tallow, while palm, coconut and palm kernel oils are the principal oils used in soap making. Chemical process in bar soap manufacturing. Batch kettle boiling method. Continuous process.
Fats and alkali are melted in a kettle, which is a steel tank that can stand three stories high and hold several thousand pounds of material. Steam coils within the kettle heat the batch and bring it to a boil. After boiling, the mass thickens as the fat reacts with the alkali, producing soap and glycerin. Boiling. The soap and glycerin must now be separated. The mixture is treated with salt, causing the soap to rise to the top and the glycerin to settle to the bottom. The glycerin is extracted from the bottom of the kettle. Salting. To remove the small amounts of fat that have not saponified, a strong caustic solution is added to the kettle. This step in the process is called strong change. The mass is brought to a boil again, and the last of the fat turns to soap. The batch may be given another salt treatment at this time, or the manufacturer may proceed to the next step. Strong change. The soap in the kettle is boiled again with added water. The mass eventually separates into two layers. The top layer is called neat soap, which is about 70% soap and 30% water. The lower layer, called nigre, contains most of the impurities in the soap such as dirt and salt, as well as most of the water. The neat soap is taken off the top. The soap is then cooled. Pitching.
SPLITTING. MIXING. splits natural fat into fatty acids and glycerin. Pumps and meters attached to the column allow precise measurements and control of the process. Molten fat is pumped into one end of the column, while at the other end water at high temperature (266°F [130°C]) and pressure is introduced. This splits the fat into its two components. The fatty acid and glycerin are pumped out continuously as more fat and water enter. The purified fatty acids are next mixed with a precise amount of alkali to form soap. Other ingredients such as abrasives and fragrance are also mixed in. The hot liquid soap may be then whipped to incorporate air. COOLING AND FINISHING. soap may be poured into molds and allowed to harden into a large slab. Then cooled in a special freezer. The slab is cut into smaller pieces of bar size, which are then stamped and wrapped. MILLING. Most toiletry soap undergoes additional processing called milling. The milled bar lathers up better and has a finer consistency than non-milled soap. The cooled soap is fed through several sets of heavy rollers (mills), which crush and knead it. Perfumes can best be incorporated at this time because their volatile oils do not evaporate in the cold mixture. After the soap emerges from the mills, it is pressed into a smooth cylinder and extruded. The extruded soap is cut into bar size, stamped and wrapped.
SPRAY DRYING. AGGLOMERATION. DRY MIXING. (1)The slurry is heated and then pumped to the top of a tower where it is sprayed through nozzles under high pressure to produce small droplets. The droplets fall through a current of hot air, forming hollow granules as they dry. (2)The dried granules are collected from the bottom of the spray tower where they are screened to achieve a relatively uniform size. (3)After the granules have been cooled, heat sensitive ingredients that are not compatible with the spray drying temperatures (such as bleach, enzymes and fragrance) are added. (4)Traditional spray drying produces relatively low density powders. leads to higher density powders, consists of blending dry raw materials with liquid ingredients. Helped by the presence of a liquid binder, rolling or shear mixing causes the ingredients to collide and adhere to each other, forming larger particles. used to blend dry raw materials. Small quantities of liquids may also be added.
LIQUID DETERGENT. SOAP PREMIX MANUFACTURE. Liquid detergents contain a combination of soap and synthetic surfactants. These are made first as a premix, after which other ingredients are blended into it. This stage simply consists of neutralizing fatty acids with either caustic soda (NaOH) or potassium hydroxide. INGREDIENT MIXING. All ingredients except the enzymes are added and mixed at a high temperature. The ingredients used in the manufacturing of liquid detergents are usually sodium tripolyphosphate, caustic soda, sulphonic acid, perfume and water. The mixture is cooled and milled, and the enzymes added in powder form. ENZYME ADDITION.
Detergents, including household cleaners, are packaged in cartons, bottles, pouches, bags or cans. The selection of packaging materials and containers involves considerations of product compatibility and stability, cost, package safety, solid waste impact, shelf appeal and ease of use. Use of packaging material made from recyclable, reusable or bio-degradable material.
The nucleophilic hydroxide anion (O is red) attacks the electrophilic carbonyl carbon (blue) in the first step of this reaction. The nucleophile approaches perpendicular to the plane of the trigonal planar carbonyl carbon and the pi electrons are displaced onto the oxygen (gold) of the carbonyl group.
The blue carbon has four bonds and is sp3 hybridized in the tetrahedral intermediate. In the second step of the mechanism, ethoxide anion (O is green) departs as an electron pair from the negative oxygen (gold) reforms the double bond of the carbonyl group.
In the last step, ethoxide anion, a strong base, removes the acidic hydrogen from the oxygen of the acetic acid. The formation of the weak base, acetate ion, in this step drives the equilibrium to the final products, the alcohol and the carboxylate anion.
Cut straight with a hand saw.
Stuck zipp washing hand pincushion
Pharmaceutical Drug used in soap
A strong alkaline solution or solid of potassium hydroxide or sodium hydroxide, made by allowing water to wash through wood ashes. It is used to make soap and drain and oven cleaners. Chemical formula: KOH or NaOH.
Sodium Hydroxide for Soap Making. RM 20/kg. To order: SMS/ Whatsapp: FB:
They strive to have the highest quality of cosmetic ingredient and at lowest rate possible.
Shea Butter- Has unique healing properties. High in triglycerides and fatty acids. The high fatty acid of shea butter also makes it an excellent additive to soap. It contains vit A and E. Coconut Oil- Healing ingredient used in soaps to produce a luxurious lather. Ingredients. Olive, Coconut, Palm and Castor Oils, Shea Butter, Cocoa Butter, Sodium Hydroxide (Lye), Mountain Spring Water, goat milk. No fragrances whatsoever.
Potassium Hydroxide is hygroscopic which is it attracts moisture, so be sure to keep it in a sealed container in a cool dry place. it can attract enough moisture from the air in the room to turn completely liquid. Always add your lye to your water. Adding water to lye can cause a volcano-like reaction. wear goggles that protect your eyes from all sides because alkali burns can cause blindness. Always wear an apron and gloves. Use rubber dish gloves. Use a heat safe container to hold your lye water, and use silicone or stainless steel tools to stir (no wood).
Industrial-scale production ; continuous process. Smaller-scale production ; batch process. There are two ways of making soap: Cold process ; under condition of room temperature. Hot process ; under condition near the boiling point.
PROCESS OF MAKING SOAP HOT PROCESS COLD PROCESS MOLD FOR INDUSTRIAL SCALE PURIFYING AND FINISHING
Commonly, the soap makers would used hot process method if they wanted to minimize the cure time to only three day air dry process. These two methods can be used for producing soap especially those who love to make handmade decorative soap. The glycerin will be left in the soap while the reaction take place for many days as soon after the soup is poured into the molds. During the hot process, the glycerin remain in the soap,however when it is applied to a high temperature,the reaction is already finished in the kettle, even before the soap is poured into the molds. In cold process of handmade soap, it uses extra fat rather than production of soap in industry. The fat is used (superfatting) to absorb the alkali, and the glycerin remained act as moisturizing agent. If the glycerin is left wet, it will cause the soap to be more softer and and less resistant mushy. Besides that, it is preferable to add lots of oil and have excess fat rather than to add lots of lye and have excess lye. Excess glycerin can be found also in hot process method. Glycerin soap can also be created by adding glycerin to the soap. It can be observed that superfatted soap is more suitable for skin however, if the fat is added too much in quantity it can leave a greasy feel to skin. Essential oils such as jojoba oil, shea butter and olive oil will remain intact and escape saponification when all lye has been consumed. Lye discount is a process where the soap makers are using less alkaline in superfatting process.
Hot process must saponifies the oil entirely before poured into the molds. The fat and hydroxide are heated and blended together at about °C which is below the boiling point until the saponification is complete. This process is the best way to use up the essential oils that cannot survive in cold process. This is just the fact that saponification has already undergo before addition of essential oils. The temperature of soap rises during saponification and numerous essential oils cook out and remain with no scent. Another benefits of hot process is it preserves the colour that we used. Hot process is more quicker than cold process method. The soap will harden and can be cut on the same day. It can be much faster by putting the soap in the refrigerator or freezer.
After hardened the soap, it will be poured into molds and in long silicon bar and cut into individual portions or poured into individual molds. Purification. At industrial scale, the soap is further purified to eliminate excess glycerol,sodium hydroxide and other compounds in the fully boiled process by boiling the crude soap curds in water and then use salt to precipitate the soap. This process proceeded in spray dryer and then into vacuum dryers. After compacted into tiny pellets or noodles, it is now ready for soap finishing which is by converting raw soap pellets into product. Fragrant and other materials are blended together in a mixer. Discharged mass will go into a refinery, vacuum chamber,cut into respective length and turn into packages. Triclosan or triclocarbon can be added in actibacterial soap.
to ensure complete melting of the fat being used. safe to use after about 12–48 hours, but is not at its peak quality for use for several weeks. Lye(sodium hydroxide) or potash(potassium hydroxide). requires exact measurements of lye and fat amounts and computing their ratio. Historically, lye used in the cold process was made from scratch using rainwater and ashes.
looks up the saponification value for each unique fat on an oil specification sheet. This value is used to calculate the exact amount of potassium hydroxide to react with the fat to form soap. Excess unreacted lye in the soap will result in a very high pH and can burn or irritate skin. In the process, the lye-fat mixture is mixed until the two phases (oils and water) are fully emulsified.
There are varying levels of trace. Depending on how additives will affect trace. After much stirring, the mixture turns to the consistency of a thin pudding. Trace corresponds roughly to viscosity. Essential oils and fragrance oils can be added. The batch is then poured into moulds. Mostly, soap molds are made of silicone or various types of plastic, although many soapmaking hobbyists may use cardboard boxes lined with a plastic film. cold-process soaps are typically cured and hardened on a drying rack for 2–6 weeks before use.
MANUFACTURING OF SOAPS & DETERGENTS
Bar soap. Powder detergent. Liquid detergent. Packaging process.
made from fats and oils. their fatty acids which are reacted with inorganic water-soluble bases. The raw materials may be pretreated to remove impurities and to achieve the color, odor and performance features desired in the finished bar. The main sources of fats are beef and mutton tallow, while palm, coconut and palm kernel oils are the principal oils used in soap making. Chemical process in bar soap manufacturing. Batch kettle boiling method. Continuous process.
Fats and alkali are melted in a kettle, which is a steel tank that can stand three stories high and hold several thousand pounds of material. Steam coils within the kettle heat the batch and bring it to a boil. After boiling, the mass thickens as the fat reacts with the alkali, producing soap and glycerin. Boiling. The soap and glycerin must now be separated. The mixture is treated with salt, causing the soap to rise to the top and the glycerin to settle to the bottom. The glycerin is extracted from the bottom of the kettle. Salting. To remove the small amounts of fat that have not saponified, a strong caustic solution is added to the kettle. This step in the process is called strong change. The mass is brought to a boil again, and the last of the fat turns to soap. The batch may be given another salt treatment at this time, or the manufacturer may proceed to the next step. Strong change. The soap in the kettle is boiled again with added water. The mass eventually separates into two layers. The top layer is called neat soap, which is about 70% soap and 30% water. The lower layer, called nigre, contains most of the impurities in the soap such as dirt and salt, as well as most of the water. The neat soap is taken off the top. The soap is then cooled. Pitching.
SPLITTING. MIXING. splits natural fat into fatty acids and glycerin. Pumps and meters attached to the column allow precise measurements and control of the process. Molten fat is pumped into one end of the column, while at the other end water at high temperature (266°F [130°C]) and pressure is introduced. This splits the fat into its two components. The fatty acid and glycerin are pumped out continuously as more fat and water enter. The purified fatty acids are next mixed with a precise amount of alkali to form soap. Other ingredients such as abrasives and fragrance are also mixed in. The hot liquid soap may be then whipped to incorporate air. COOLING AND FINISHING. soap may be poured into molds and allowed to harden into a large slab. Then cooled in a special freezer. The slab is cut into smaller pieces of bar size, which are then stamped and wrapped. MILLING. Most toiletry soap undergoes additional processing called milling. The milled bar lathers up better and has a finer consistency than non-milled soap. The cooled soap is fed through several sets of heavy rollers (mills), which crush and knead it. Perfumes can best be incorporated at this time because their volatile oils do not evaporate in the cold mixture. After the soap emerges from the mills, it is pressed into a smooth cylinder and extruded. The extruded soap is cut into bar size, stamped and wrapped.
SPRAY DRYING. AGGLOMERATION. DRY MIXING. (1)The slurry is heated and then pumped to the top of a tower where it is sprayed through nozzles under high pressure to produce small droplets. The droplets fall through a current of hot air, forming hollow granules as they dry. (2)The dried granules are collected from the bottom of the spray tower where they are screened to achieve a relatively uniform size. (3)After the granules have been cooled, heat sensitive ingredients that are not compatible with the spray drying temperatures (such as bleach, enzymes and fragrance) are added. (4)Traditional spray drying produces relatively low density powders. leads to higher density powders, consists of blending dry raw materials with liquid ingredients. Helped by the presence of a liquid binder, rolling or shear mixing causes the ingredients to collide and adhere to each other, forming larger particles. used to blend dry raw materials. Small quantities of liquids may also be added.
LIQUID DETERGENT. SOAP PREMIX MANUFACTURE. Liquid detergents contain a combination of soap and synthetic surfactants. These are made first as a premix, after which other ingredients are blended into it. This stage simply consists of neutralizing fatty acids with either caustic soda (NaOH) or potassium hydroxide. INGREDIENT MIXING. All ingredients except the enzymes are added and mixed at a high temperature. The ingredients used in the manufacturing of liquid detergents are usually sodium tripolyphosphate, caustic soda, sulphonic acid, perfume and water. The mixture is cooled and milled, and the enzymes added in powder form. ENZYME ADDITION.
Detergents, including household cleaners, are packaged in cartons, bottles, pouches, bags or cans. The selection of packaging materials and containers involves considerations of product compatibility and stability, cost, package safety, solid waste impact, shelf appeal and ease of use. Use of packaging material made from recyclable, reusable or bio-degradable material.
The nucleophilic hydroxide anion (O is red) attacks the electrophilic carbonyl carbon (blue) in the first step of this reaction. The nucleophile approaches perpendicular to the plane of the trigonal planar carbonyl carbon and the pi electrons are displaced onto the oxygen (gold) of the carbonyl group.
The blue carbon has four bonds and is sp3 hybridized in the tetrahedral intermediate. In the second step of the mechanism, ethoxide anion (O is green) departs as an electron pair from the negative oxygen (gold) reforms the double bond of the carbonyl group.
In the last step, ethoxide anion, a strong base, removes the acidic hydrogen from the oxygen of the acetic acid. The formation of the weak base, acetate ion, in this step drives the equilibrium to the final products, the alcohol and the carboxylate anion.
Cut straight with a hand saw.
Stuck zipp washing hand pincushion

Pharmaceutical Drug used in soap. - ppt download

Introductory Guide to Therapeutic Drug Monitoring: What's Ne

Soaps and detergents Dr. surendran parambadath
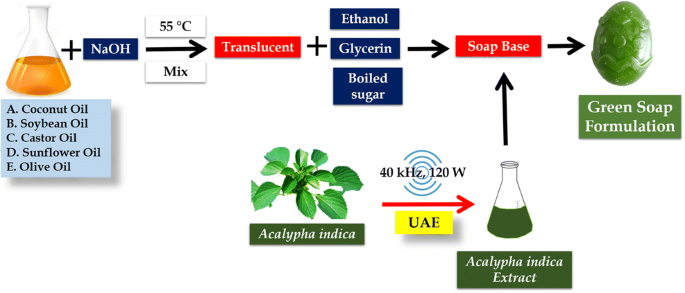
Green soap formulation: an insight into the optimization of preparations and antifungal action

TOILETRIES SOAPS.. - ppt download

Explore Treatment - POMALYST® (pomalidomide)

Pharmaceutical Drug used in soap. - ppt download

Case Presentation in SOAP Format

Presentation on Preparation of soap

SOAP notes

Pharmaceuticals Solutions dosage form

SOAP.pptx
from
per adult (price varies by group size)